Scientists Develop a Quantitative Proteomics Platform for Revealing Bioactive Mechanism of Natural Products through Activity-based Protein Profiling Strategy
Natural products have played a crucial role in the development of new therapeutic agents. However, the target identification for natural products is still challenging due to the structural complexity and the lack of efficient biochemical methods. Recently, scientists led by
Prof. Youli Xiao from the Institute of Plant Physiology and Ecology, CAS Center for Excellence in Molecular Plant Sciences made progress in the target identification and molecular mechanism of a series of bioactive natural products from plants.
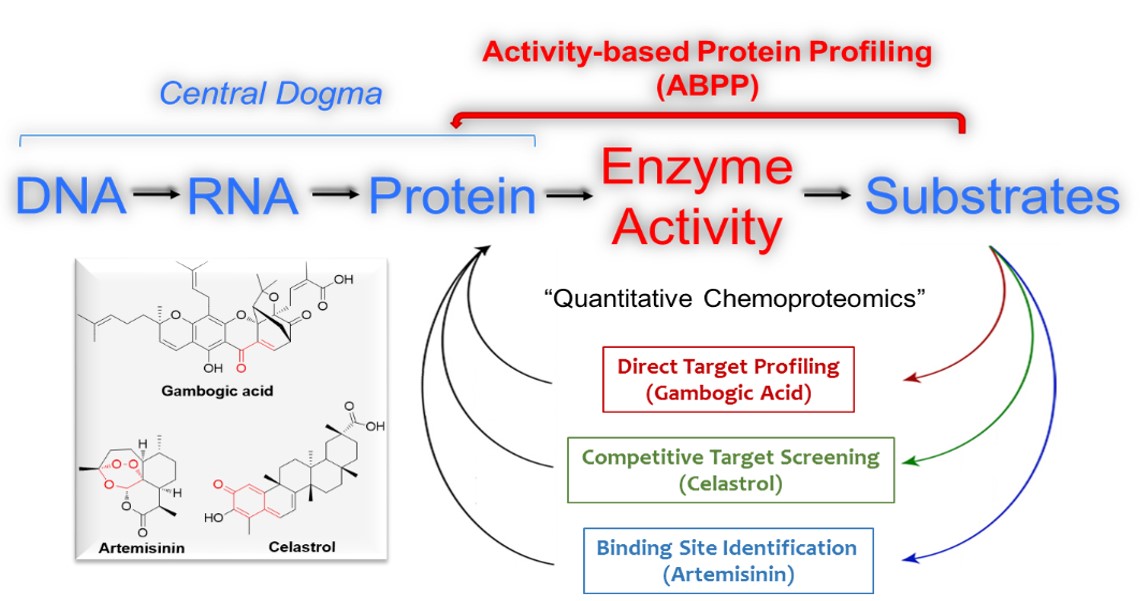
In the post-genome era, a widely applicated strategy for annotating of biochemical pathways is based on genome sequencing and bioinformatics analysis. Activity-based protein profiling (ABPP) is a functional proteomic technology that uses small molecule-based chemical probes that react with mechanistically related classes of enzymes. Unlike the conventional method, ABPP monitors the activity of the proteins (enzymes) directly, rather than being limited to genome or transcriptome.
As an extension of target profiling and mechanism studies on artemisinin (
ACS Chem. Biol., 2016), they reported the characterization of the artemisinin binding site of Plasmodium falciparum translationally controlled tumor protein (TCTP) via “capture and release” strategy utilizing an artemisinin activity-based probe. In addition, they developed a quantitative proteomics platform for the target profiling of gambogic acid and celastrol, two natural products with potent anti-cancer and anti-inflammatory activities. By utilizing a synthetic gambogic acid-derived probe, they mapped the target profiles of gambogic acid in human cancer cells and RPS27A was identified as a potential physiological related target of gambogic acid; unlike gambogic acid, celastrol alkylates target proteins through a unique covalent and reversible mechanism. Therefore, a competitive-ABPP strategy utilizing a cysteine-reactive probe was applied to screening the potential targets of celastrol. Bioinformatics analysis suggested that celastrol could induce cancer cell apoptosis by interfering cellular thiol redox metabolism.
The work entitled “Competitive profiling of celastrol targets in human cervical cancer HeLa cells via quantitative chemical proteomics” has been published online in Molecular BioSystems on November 7th.
The work entitled “Global profiling of cellular targets of gambogic acid by quantitative chemical proteomics” has been published online in Chemical Communications on November 9th.
The work entitled “Characterization of the Artemisinin Binding Site for Translationally Controlled Tumor Protein (TCTP) by Bioorthogonal Click Chemistry” has been published online in Bioconjugate Chemistry on November 16th.
The research was funded by the National Nature Science Foundation of China, Chinese Academy of Sciences and Shanghai Committee of Science and Technology.
CONTACT:
Youli Xiao, Ph.D., Principal Investigator, Institute of Plant Physiology and Ecology, CAS Center for Excellence in Molecular Plant Sciences Chinese Academy of Sciences, Shanghai 200032, China Tel.: 86-21-54924226, Fax: 86-21-54924226, E-mail:
ylxiao@sibs.ac.cn